The Role of LIMS QC and LES
Laboratory Digital Transformation: The Role of LIMS QC and LES
In today's industrial and scientific research fields, LIMS Quality Control (QC) and Laboratory Execution Systems (LES) have become the core of laboratory digital transformation. They not only enhance the efficiency and accuracy of laboratories but also ensure compliance and innovation.

I. The Cornerstones of Laboratory Digital Transformation: LIMS QC and LES
LIMS QC: The Guardian of Quality Control
LIMS Quality Control (QC) is the process of ensuring that products and services meet specific quality standards and consumer needs. In a laboratory setting, LIMS QC covers the entire process from sample receipt to analysis, result approval, and certificate generation.
The key activities of LIMS QC include:
Sample Management: Establishing a sample folder from receipt to the end of its lifecycle, tracking any discrepancies.
Testing and Analysis: Conducting in-house and outsourced testing and analysis, as well as additional reviews and analysis.
Results Management: Defining measurement parameters for each type of analysis and managing all types of results.
Certificate Generation: Closing the analysis or control requests based on compliance and generating a Certificate of Analysis (CoA).
LES: The Automated Executor of Laboratory Operations
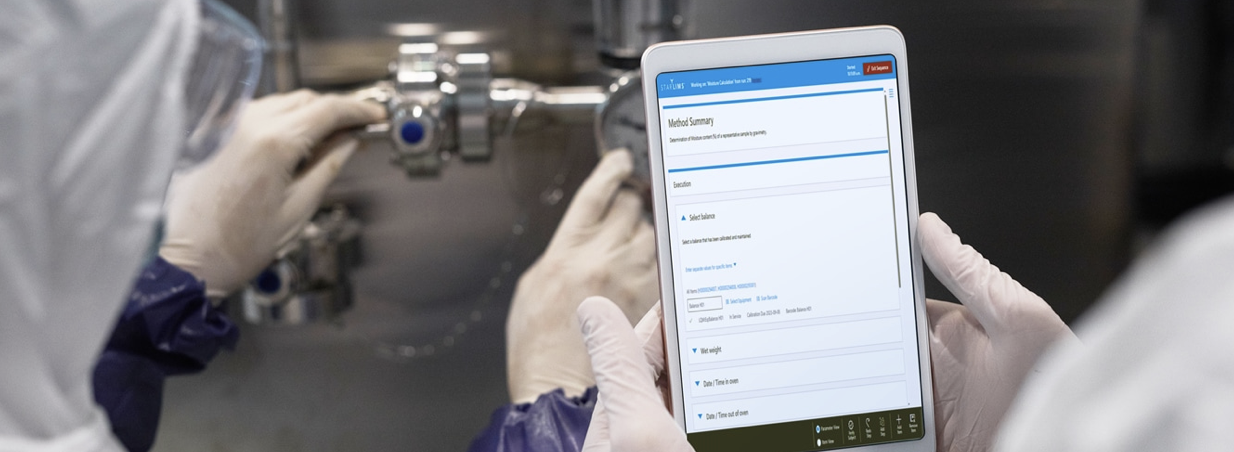
Laboratory Execution Systems (LES) aim to guide and automate the real-time execution of laboratory procedures. They ensure that technicians follow standard methods by providing step-by-step instructions, thereby enforcing protocol compliance, reducing human error, and supporting quality control.
The main features of LES include:
Execution Protocol Management: Managing execution protocols by guiding users through detailed instructions for each step of a procedure.
Real-Time Data Collection: Collecting real-time data and tracking every step in the experimental process.
Quality Control: Conducting quality control through verification and approval of results, as well as alerts or reminders for non-compliance.
The main difference between LES and LIMS QC is that LES focuses more on the execution and monitoring of processes, while LIMS QC involves the entire strategy and activities of quality control.
II. The Synergy of LIMS QC and LES
In the process of laboratory quality management, LIMS QC and LES are not isolated systems but complementary tools that work together to enhance laboratory efficiency and compliance. LIMS QC ensures that laboratory activities meet quality standards, while LES ensures that these activities are executed according to established processes and standards. This synergy has been highlighted in popular discussions on Zhihu, where many industry experts and laboratory workers have emphasized the importance of using both systems in conjunction.
Enhancing Efficiency and Accuracy
The combined use of LIMS QC and LES can significantly improve the efficiency and accuracy of laboratory work. LIMS QC defines quality standards and parameters, making data easily retrievable and analyzable, while LES ensures that experimental steps are carried out according to established SOPs, reducing errors. This combination allows laboratories to respond quickly to changes while maintaining high standards of experimental quality.
Ensuring Compliance and Traceability
In industries such as pharmaceuticals and chemicals, compliance and traceability are crucial. The combined use of LIMS QC and LES can ensure the integrity and accuracy of experimental data, meeting the requirements of regulatory authorities. The audit trail function of LIMS QC and the protocol execution monitoring of LES together ensure the transparency and traceability of the experimental process.
Promoting Innovation and Knowledge Sharing
The collaborative features of LIMS QC and the standardized operations of LES provide a platform for laboratory staff to share knowledge and best practices. This knowledge sharing not only accelerates the innovation process but also improves the work efficiency of the entire team.
Laboratory quality management is a complex process involving the integration and optimization of multiple systems. LIMS QC and LES, as the two main pillars of this transformation, work together to provide a powerful tool for improving efficiency, ensuring compliance, promoting innovation, and sharing knowledge. As technology advances and laboratory needs continue to evolve, LIMS QC and LES will continue to develop, offering more value to laboratory workers. By understanding the functions and advantages of these systems, laboratories can better plan their quality management path to adapt to future challenges.